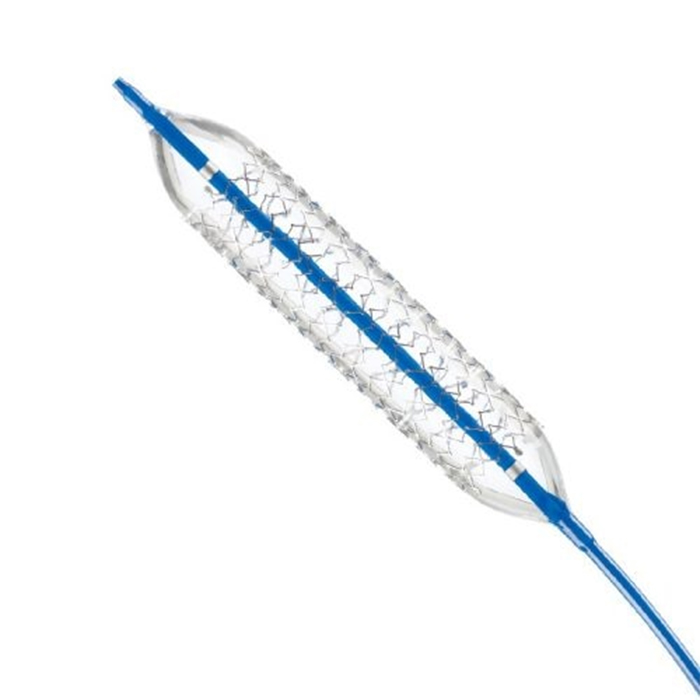
Description
MARKERS
ENVIRONMENTAL
The Visi-Pro balloon-expandable peripheral stent is indicated for use in occlusions, lesions at high risk of sudden closure after percutaneous transluminal angioplasty (PTA), or lesions believed to be at high risk of post-PTA stenosis in the following arteries:
- Common and external iliac, subclavian
- Kidney
Stenting aims to improve and maintain the arterial lumen diameter.
BILIARY
The Visi-Pro balloon expandable peripheral stent is indicated for use as the palliative treatment of malignant neoplasms in the biliary tree. Not all indications for the VISI-PRO BALLOON EXPANDABLE PERIPHERAL AND BILIARY STENT have been validated globally, please refer to the complete Directions for Use guide for approved Use indication in your geography.
PRODUCT DETAILS
PREMIUM GÖRÜNÜRLÜK
- Distinctive 0.035′ balloon expandable stent with radiopaque markings provides visibility during and after stent placement.
SOLID RADIAL STRENGTH AND FLEXIBILITY
- Patented design balances strength and flexibility.
PRECISE PLACEMENT
- Minimal dilatation of healthy tissue is dependent on balloon-to-stent placement.
- Provides correct catheter marker alignment to balloon taper.
- The progressive stent design provides minimal shortening.