Description
MARKERS
ENVIRONMENTAL
The ParaMount Mini GPS balloon expandable peripheral stent is indicated for use in occlusions, lesions at high risk of sudden closure or threatened closure after percutaneous transluminal angioplasty (PTA), or lesions believed to be at high risk of stenosis following PTA in the renal arteries. Stenting aims to improve and maintain the arterial lumen diameter.
BILIARY
The ParaMount Mini GPS balloon expandable peripheral stent is indicated for use as the palliative treatment of malignant neoplasms in the biliary tree.
Not all indications for the PARAMOUNT MINI GPS BALLOON EXPANDABLE PERIPHERAL AND BALLAIY STENT have been validated globally, please refer to the complete Directions for Use guide for approved Use indication in your geography.
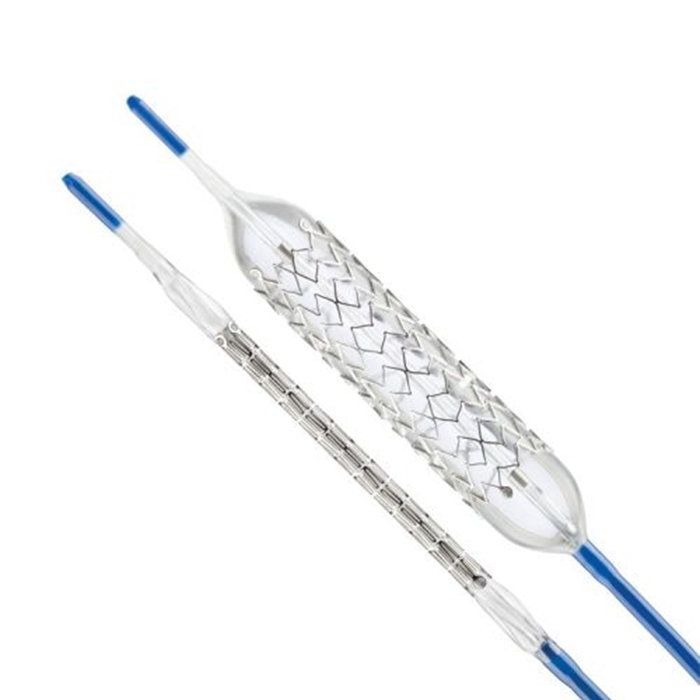
PRODUCT DETAILS
HIGH VISIBILITY
- GPS radiopaque tantalum markers are embedded at each end of the stent for improved procedural accuracy and visibility.
- GPS marks define the near and far edges as the stent expands.
- GPS technology ensures that the stent remains visible under fluoroscopy to confirm stent placement.

GPS Markers
Position of GPS radiopaque markers at the end of the ParaMount Mini GPS stent.
LOW PROFILE SYSTEM
- Available on a 0.014″ and 0.018″ guidewire compatible balloon catheter.
- Compatible with 6 F or 7 F guide catheter.
- It is designed for flexibility when traveling to a target lesion.
STRENGTH AND FLEXIBILITY
- The unique cell geometry provides greater radial strength and support.
SENSITIVE
- Medtronic’s proprietary Micro-Grip™ technology ensures proper centering of the stent prior to expansion.
- Stent shows minimal shortening at recommended expansion diameters